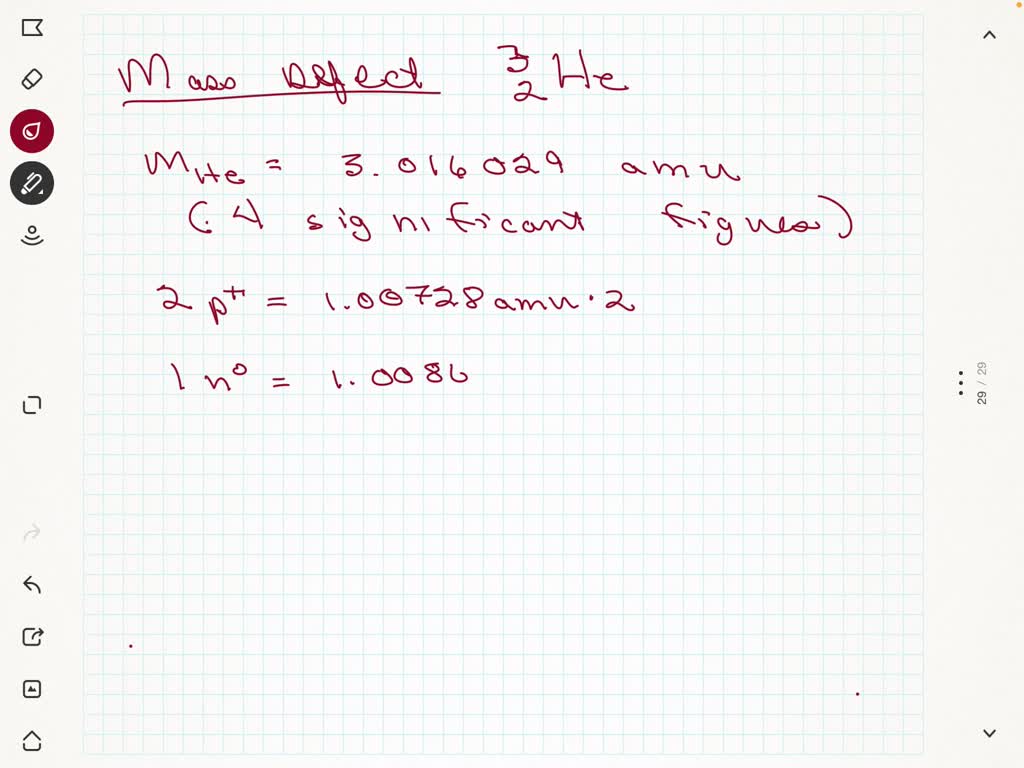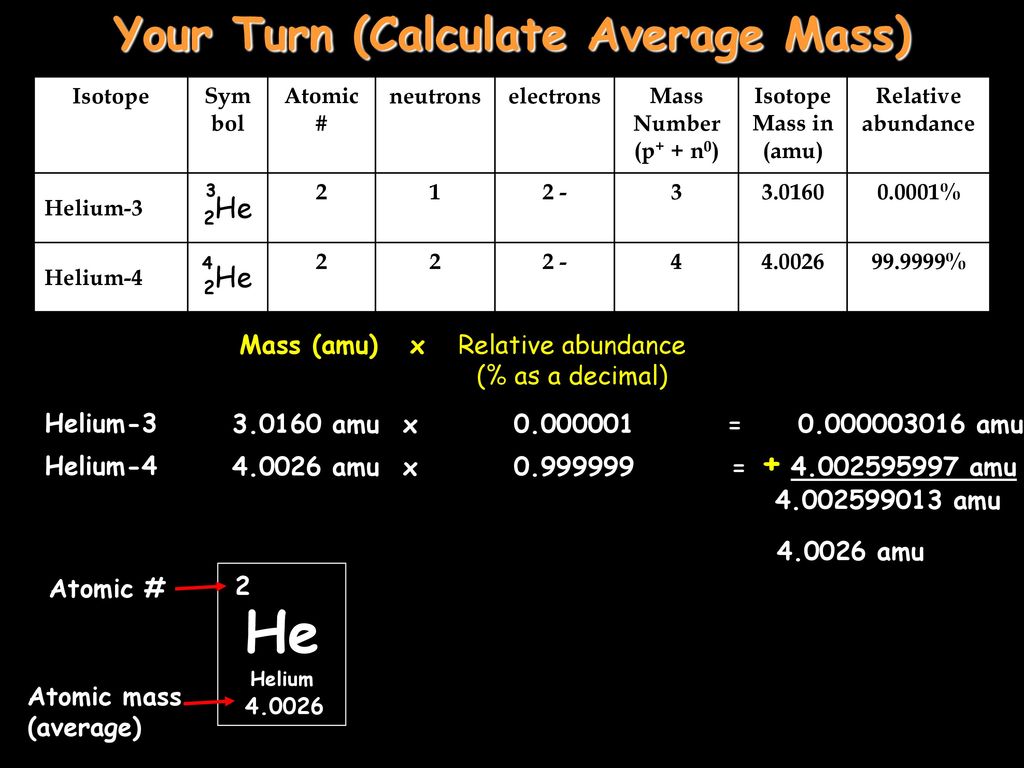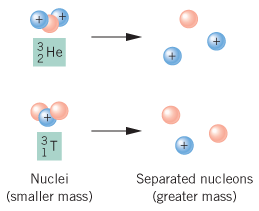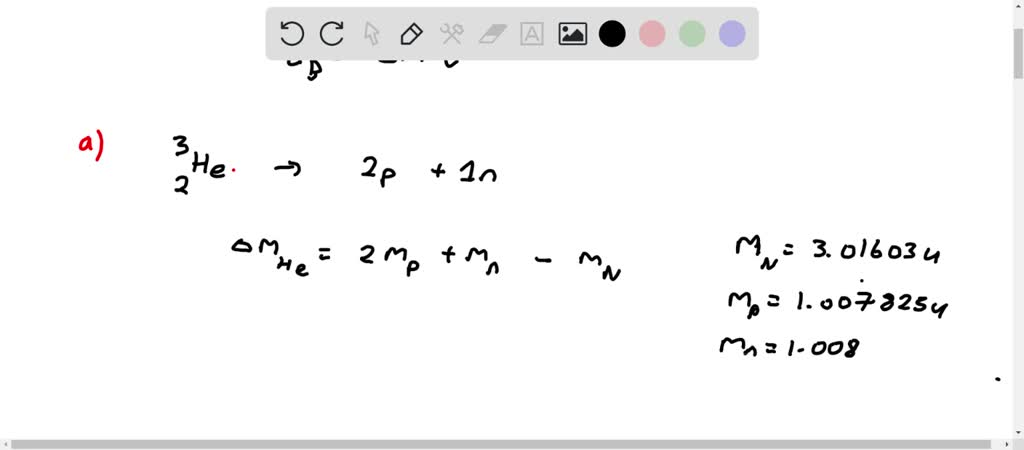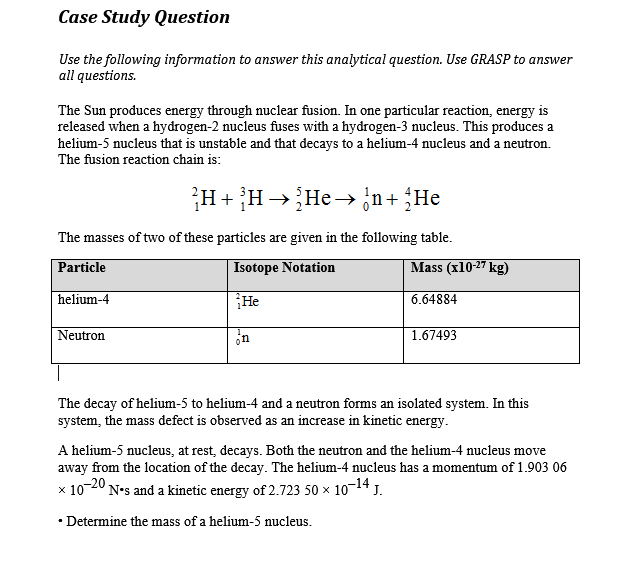
A helium 5 decays into helium-4 and neutron. Helium-4 nucleus has a momentum = 1.903 06 × 10–20 N•s & kinetic energy of 2.723 50 × 10–14 J. Helium-4 mass has a

The positions x and y for m1, m2, and the Reduced Mass on the Helium-3... | Download Scientific Diagram

The density of helium is 0.1784 kg/m^3 STP. If a given mass of helium STP is allowed to to 1.400 times of its initial volume by changing P and T, compute its

Calculate the binding energy of helium nucleus `(._(2)^(4)He)` and express the quantity in MeV and - YouTube