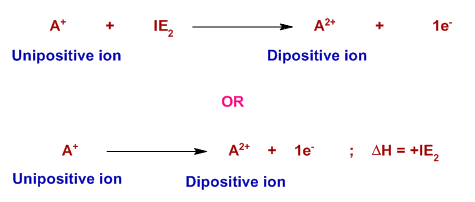
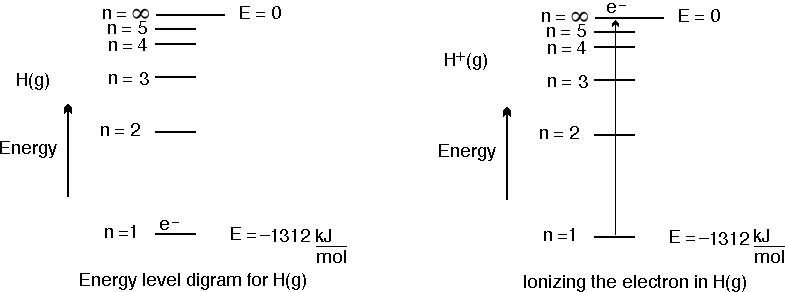
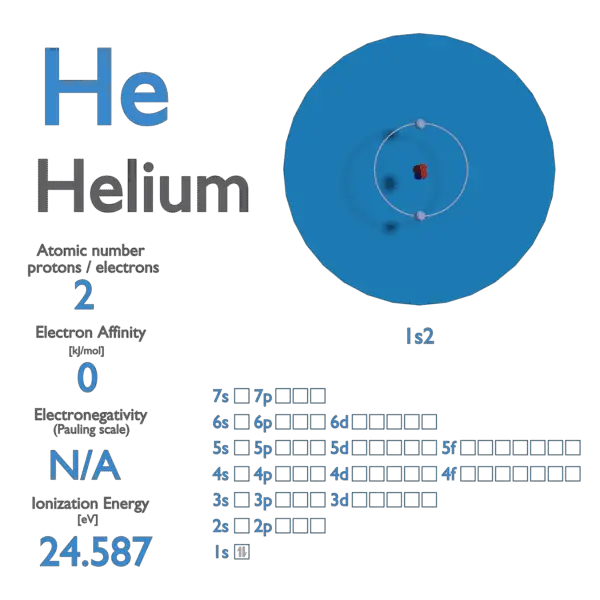
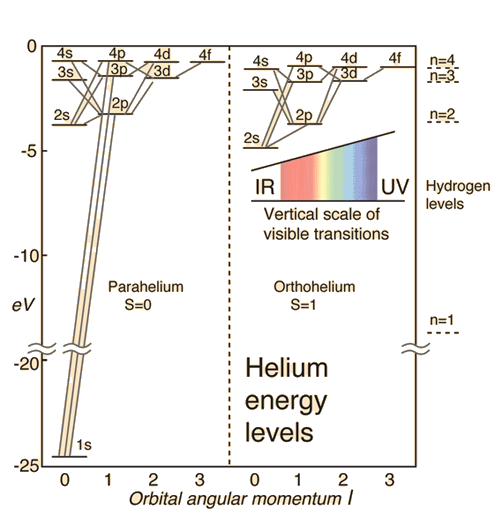
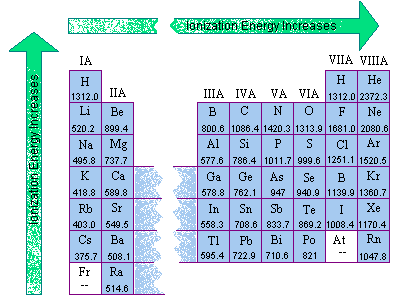
Ionization Energies Originated 11/20/11 Last revision 05/19/12 Mike Jones Pisgah High School Canton NC. - ppt download

The ionisation energy of hydrogen atom is 13.6 eV, the ionisation energy of helium atom would be (1988) (a) 13.6 eV (b) 27.2 eV (c) 6.8 eV (d) 54.4 eV

Appearance intensity versus ionization energy for nitrogen and helium... | Download Scientific Diagram
Helium Chemical Element First Ionization Energy Stock Vector (Royalty Free) 1220935573 | Shutterstock

The arrangement of electrons in an atom helps determine the properties and behavior of that atom. - ppt download
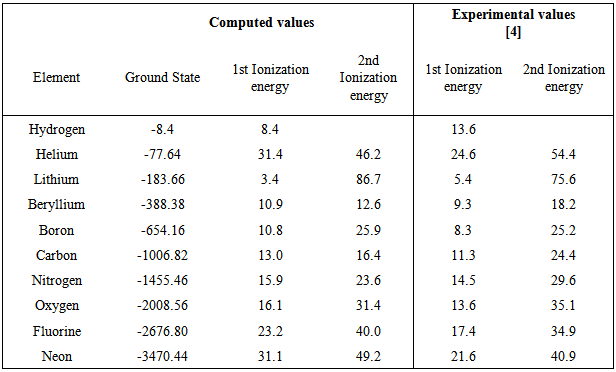
Computation of the First and Second Ionization Energies of the First Ten Elements of the Periodic Table Using a Modified Hartree-Fock Approximation Code